

As with atopic dermatitis and psoriasis, the treatment of AA with systemic medications is merited, and it has been shown that quality of life in AA can be restored with successful treatment. The results of large clinical trials using different JAK inhibitors in AA will begin to answer this and other questions, although many thousands of treated patients and long surveillance periods will be necessary to fully assess malignancy risk, especially for cancers that have long latency periods.ĪA is a common disorder that is often associated with poor quality of life, depression, and anxiety. 9 As with the tumor necrosis factor-α inhibitors, it may be that there are different risks of JAK inhibitor treatment in different disease states. 7, 8 It is interesting that during the controlled clinical trials of tofacitinib in ulcerative colitis (1220 patients, up to 52 weeks of treatment), there were no cases of solid organ malignancy or lymphoma.
ALOPECIA UNIVERSALIS VS ALOPECIA AREATA SKIN
In phase 2, phase 3, and long-term extension studies of baricitinib and tofacitinib treatment of rheumatoid arthritis, the rate of all malignancies (excluding nonmelanoma skin cancer) was 0.8 per 100 patient years with baricitinib (95% confidence interval, 0.6 to 1.0) and 0.85 per 100 patient years with tofacitinib (95% confidence interval, 0.7 to 1.02), similar to what is observed for rheumatoid arthritis, in general.
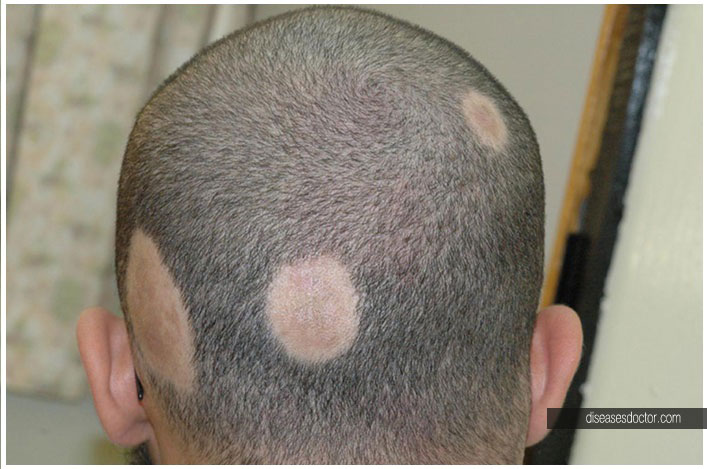
Like the tumor necrosis factor-α inhibitors (eg, adalimumab), tofacitinib and baricitinib carry a black box warning regarding infection and malignancy risk. While the potential risks of JAK inhibition must be discussed with every patient, they are especially important to consider in a patient with a history of cancer. To our knowledge, this is the first report of a patient with AA successfully treated with baricitinib monotherapy. Here we describe a case of a woman with severe AA, with complete scalp and near-complete body hair loss, who experienced complete hair regrowth with baricitinib 4 mg daily. 6 Baricitinib is a relatively new JAK1/2 inhibitor that was recently approved for the treatment of rheumatoid arthritis in Europe and Japan at doses of 2 and 4 mg daily and in the United States at 2 mg daily. 3, 4, 5 There is a report of a patient with AA and chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome, whose AA was successfully treated during treatment of CANDLE syndrome with high-dose baricitinib, 7 to 11 mg daily, in addition to prednisone.
ALOPECIA UNIVERSALIS VS ALOPECIA AREATA SERIES
In particular, the JAK1/3 inhibitor, tofacitinib, and the JAK1/2 inhibitor, ruxolitinib, have been found in larger series of patients to be effective for severe disease. 2 Cell signaling via interferon-γ and interleukin-15 occurs via the Janus kinase (JAK) family of enzymes, and JAK inhibitors have been found to reverse disease. 1 In a murine model of AA, up-regulation of interleukin-15 in hair follicles leads to recruitment and activation of natural killer gene 2D–expressing CD8 T cells, which, in turn, produce interferon-γ, further activating the hair follicle epithelial cells. Alopecia areata (AA) is a nonscarring form of hair loss characterized by well-defined patches of alopecia, typically involving the scalp, and less commonly by near-complete or complete scalp and body hair loss.


 0 kommentar(er)
0 kommentar(er)
